Abstract
Genetic and Clinical Background The clinical outcome of Core Binding Factor Leukemia (CBFL) has been reported to be influenced by KIT mutations, that was frequent in CBFL, and by co-occurring different spectra of cooperating mutations. In addition, gene expression studies found KIT to be highly expressed in CBFL regardless of its mutational status. In vitro experiments revealed that Midostaurin (Mido) is effective in inhibiting both wild type (WT) and a range of KIT mutants. In vivo studies demonstrate the efficacy in KIT-positive malignancies such as Aggressive Systemic Mastocytosis, Mast Cell Leukemia, and SM with Associated Hematological Neoplasm.
Therefore, we designed a Phase II trial to evaluate the safety and efficacy of Mido, in association with Intensive Chemotherapy (IC), in CBFL regardless of KIT mutational status. (EudraCT Number 2017-002094-18; ClinicalTrials ID: NCT 03686345). 34 adult patients with t(8;21) (n=16) and inv(16) (n=18) were enrolled between December 2018 to July 2022.
Method Patients with de-novo CBFL received standard induction therapy with an anthracycline containing regimen ("7+3"-like) + Mido, three cycles of post-remission consolidation chemotherapy with high-dose cytarabine + Mido, and 12 months of Mido as Maintenance.
At diagnosis, 32 out of 34 patients were studied by a comprehensive NGS panel targeting 40 DNA genes and 29 RNA fusion driver genes associated with major myeloid disorders (Oncomine Myeloid Research Assay).
Aim To study the clonal evolution of CBFLs, NGS and MRD status (performed by qPCR and high-resolution multicolor flow cytometry) were assessed at established check-points during consolidation, maintenance therapy, and at relapse.
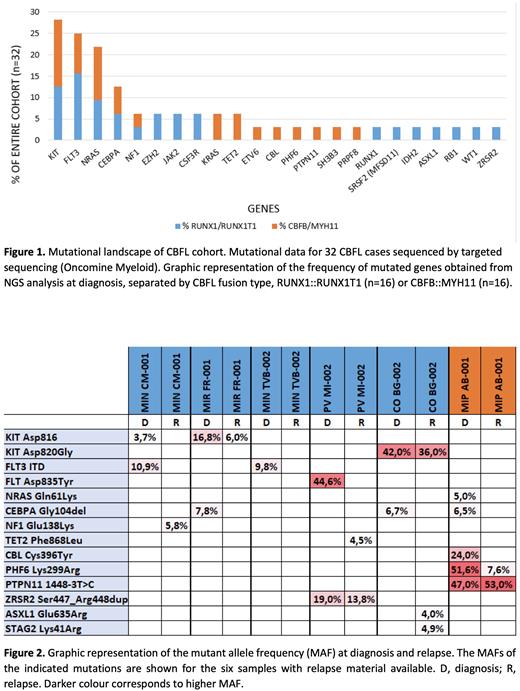
ResultsKIT was the most frequently mutated gene (28%) and there was an enrichment of FLT3 and NRAS (Figure 1). Additionally, the spectrum of recurrent mutations was different, as codon 816 KIT mutations and FLT3-ITD were common only in RUNX1::RUNX1T1 cases.
Overall, the CR rate was 97%. At a median follow-up of 18 months (range 2-43 months), we recorded a RI of 37.5 %, an OS of 85 %, and a DFS of 60%.
We performed targeted next generation sequencing for six out of 12 relapsed samples in the cohort t(8;21) (n=5) and inv(16) (n=1), showing loss, retention or gain of somatic mutations (Figure 2). Three out of six relapsed patients showed at diagnosis a CEBPA mutation (Gly104del) co-occurring with KIT in RUNX1::RUNX1T1 cases and with NRAS in CBFB::MYH11.
Conclusion In patients with CBFL, we found FLT3 and/or KIT mutations actionable by a regimen consisting of intensive chemotherapy and Mido in 47% of cases.
In two out of three relapsed cases the KIT mutation is maintained, although frequency of the mutant allele is lower at relapse, while all FLT3 and NRAS mutated patients lose the mutation upon disease relapse.
Disclosures
Cairoli:Novartis, Celgene: Speakers Bureau; Gilead Sciences: Other: Travel, Accommodations; Celgene, AbbVie, Daiichi Sankyo, Novartis: Consultancy.
OffLabel Disclosure:
Midostaurin in the treatment of CBFL
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal